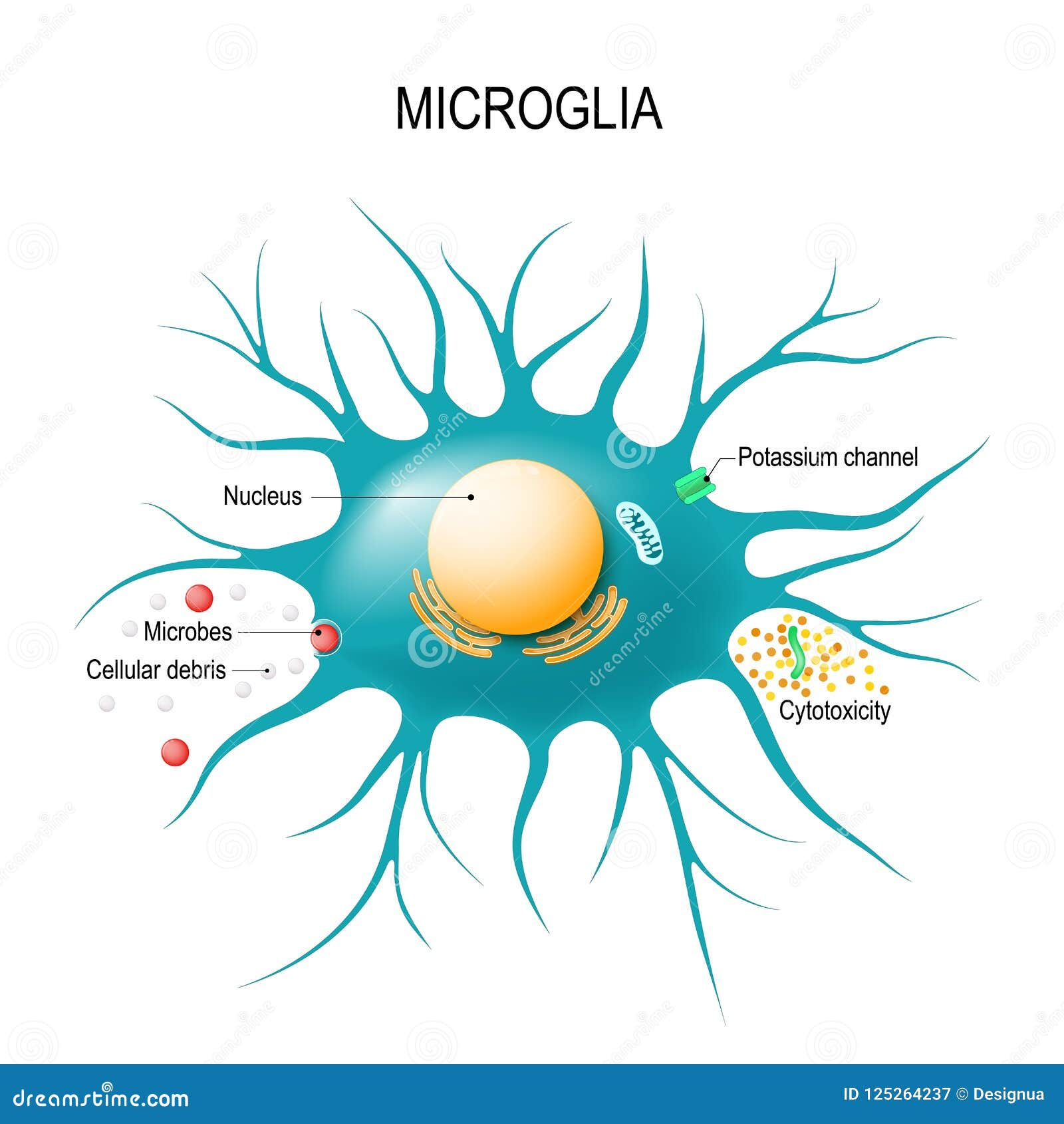
Microglial cells are pivotal players in the brain’s immune system, actively safeguarding our cerebral health. As researchers delve deeper into Alzheimer’s research, the critical role these immune cells play in neurodegenerative diseases has come to the forefront. Led by innovative scientists like Beth Stevens, the understanding of microglia function is evolving, revealing their dual role in both protecting and potentially harming neuronal health. These cells patrol the brain’s environment, clearing away debris and regulating synaptic connections essential for cognitive processes. However, when their function goes awry, it can lead to pathological conditions, making microglial cells a focal point for novel therapies aimed at treating the millions affected by Alzheimer’s and similar disorders.
In the intricate landscape of the central nervous system, glial cells—specifically microglia—act as the brain’s primary defenders, embodying the immune response within this vital organ. These brain-resident immune cells are becoming increasingly prominent in discussions surrounding neurodegenerative conditions, particularly those impacting memory and cognition, like Alzheimer’s. The groundbreaking work of researchers, such as Beth Stevens, is reshaping our comprehension of how these cells function, acknowledging their essential role in maintaining neural integrity while also highlighting potential pitfalls in their activity. By investigating the dynamics of glial biology, scientists are unlocking new pathways for therapies that could significantly alter the trajectory of Alzheimer’s disease and similar ailments. Thus, the exploration of microglial behavior not only enriches our knowledge of brain function but also propels forward the quest for effective treatments in neurology.
Understanding Microglial Cells in Brain Health
Microglial cells are essential components of the brain’s immune system, playing a crucial role in maintaining neural health. These specialized immune cells are continuously monitoring the brain environment, providing support for neuronal function, and orchestrating the response to any form of injury or disease. In the realm of Alzheimer’s research, understanding microglia’s functionality has become increasingly important, as these cells may either protect against neurodegeneration or contribute to its progression by aberrantly pruning synapses.
Research led by Beth Stevens has significantly illuminated the roles of microglial cells, showcasing their dual nature in neurodegenerative diseases such as Alzheimer’s and Huntington’s. Stevens’ findings highlight that while microglia help clear out dead neurons and support healthy synapse formation, their overactivity or dysfunction can result in detrimental neuronal loss. This compelling evidence underscores the complexity of microglial function and their pivotal role in the brain’s immune responses, emphasizing the need for continued investigation into their mechanisms.
Beth Stevens: Pioneering Research in Neurodegenerative Diseases
Beth Stevens has established herself as a leading figure in the study of microglia and their implications in neurodegenerative diseases. With substantial support from the NIH, her research has unraveled the intricate connections between microglial activity and diseases like Alzheimer’s. Stevens emphasizes the importance of basic science, noting that seemingly disconnected studies can ultimately yield significant insights into human health challenges. Her innovative approaches have pioneered new avenues for investigating brain immune functions and their impact on neurodegeneration.
By focusing on how microglial cells contribute to synaptic pruning during normal brain development, Stevens has significantly altered the perspective on neuroinflammatory processes. Her groundbreaking work has initiated movements towards establishing biomarkers for early detection of Alzheimer’s disease, demonstrating the crucial link between microglia and neuronal health. As these insights grow, they pave the way for novel therapeutic strategies aimed at modulating microglial activity to combat the effects of neurodegenerative diseases.
The Role of Microglia in Alzheimer’s Disease
Microglia are increasingly recognized as pivotal players in the pathology of Alzheimer’s disease. These brain-resident immune cells engage in processes such as synaptic pruning, crucial for normal cognitive function. However, in the context of Alzheimer’s, dysregulated microglial responses can exacerbate synaptic loss and neuronal death, catalyzing disease progression. A deeper understanding of microglial function is essential for innovating treatment approaches that could mitigate their detrimental effects in neurodegenerative diseases.
Research spearheaded by Stevens highlights that aberrant microglial activation may lead to both neuroinflammation and improper synaptic pruning, fueling the spiraling pathology characteristic of Alzheimer’s. Her studies suggest that therapeutic interventions targeting microglia could help restore balance and promote neuroprotection. As scientists delve into the complexities of microglial behavior, the hope is to uncover strategies that harness these cells’ protective capabilities while mitigating their role in the progression of Alzheimer’s disease.
Importance of Basic Science in Neurodegenerative Research
Beth Stevens emphasizes that foundational research is vital for breakthroughs in understanding diseases such as Alzheimer’s. Her journey illustrates how basic science fosters innovation, allowing researchers to investigate neural mechanisms and potential therapeutic targets. As the field of neurodegenerative research evolves, it is evident that the complexities uncovered through fundamental studies lay the groundwork for clinical advancements, fostering a deeper comprehension of neuronal interactions.
Scientific curiosity drives the exploration of seemingly abstract concepts, which may eventually translate into real-world applications. The detailed studies conducted by Stevens and her team open up new avenues for understanding the intricate brain immune system and its interplay with neurodegenerative diseases. Thus, sustaining investment in fundamental research is essential for the continual progress necessary for future discoveries and applications in treating conditions like Alzheimer’s.
Neuroinflammation and Its Impact on Brain Health
Neuroinflammation plays a critical role in various neurodegenerative diseases, with microglial cells acting as the primary mediators. These cells react to injury and disease by releasing pro-inflammatory cytokines, which can have both protective and harmful effects. In the context of Alzheimer’s disease, the chronic activation of microglia leads to sustained inflammation, which is associated with synaptic disruption and neurodegeneration. Understanding the fine balance of microglial response is crucial in addressing the disease’s progression.
The transformative research conducted by Stevens highlights the importance of regulating neuroinflammatory processes to protect against neurodegeneration. By identifying the factors that trigger microglial activation, strategies can be developed to attenuate inflammation while preserving the cells’ protective roles. This underscores the necessity of a coordinated approach in Alzheimer’s research, linking neuroinflammation with therapeutic interventions to enhance brain health.
Innovative Biomarkers Derived from Microglial Research
Innovative research exploring microglial function has the potential to yield new biomarkers pertinent to neurodegenerative diseases. Understanding how microglial cells respond to pathological processes offers insights into early detection mechanisms for conditions such as Alzheimer’s. By linking specific microglial activities to disease progression, researchers aim to facilitate earlier diagnosis and improved therapeutic alignment, enhancing patient care and treatment outcomes.
Through the identification of microglial-related biomarkers, studies can establish connections between pathological mechanisms and clinical manifestations of Alzheimer’s disease. This advancement is pivotal for personalized medicine, allowing for tailored interventions that address individual patient needs based on their unique microglial responses. The continuous exploration of these biomarkers illustrates the ongoing commitment to integrating cutting-edge science with clinical applications.
Collaborative Efforts in Alzheimer’s Research
Collaboration across various fields has become increasingly vital in Alzheimer’s research, uniting neuroscientists, immunologists, and clinicians to tackle the challenges posed by neurodegenerative diseases. Stevens’ work exemplifies the strength of interdisciplinary approaches, where insights from basic science and clinical practice converge to advance knowledge around neuroinflammation and microglial function. Such collaborations not only enhance research quality but also accelerate the application of findings to real-world therapeutic scenarios.
Strategic partnerships also promote resource sharing, enabling researchers to delve deeper into complex mechanisms underlying brain health. By working together, scientists can expand their intellectual horizon and integrate diverse perspectives, fostering innovation. This collaborative spirit is essential for making strides in Alzheimer’s research, with the ultimate goal of translating discoveries into effective treatments that improve outcomes for millions affected by neurodegenerative diseases.
Future Directions in Microglial Research
The future of microglial research holds promising potential for uncovering novel therapeutic strategies against diseases like Alzheimer’s. With ongoing studies aimed at understanding microglial behavior and the factors driving their activation, researchers are poised to develop new methods for controlling neuroinflammation. By harnessing the capabilities of these cells, innovative treatments could emerge that not only halt disease progression but also promote neuroprotection and recovery.
Emerging technologies such as single-cell RNA sequencing and advanced imaging techniques are providing unprecedented insights into the dynamic roles of microglia. These advances will likely pave the way for tailored therapeutic approaches that can selectively modulate microglial responses based on individual patient profiles. As the field continues to advance, the integration of these new tools will enhance our understanding and treatment of neurodegenerative diseases, building on the crucial work led by scientists like Beth Stevens.
Frequently Asked Questions
What role do microglial cells play in Alzheimer’s research?
Microglial cells are crucial in Alzheimer’s research as they function as the brain’s immune system. They are involved in clearing dead or damaged cells and pruning synapses, which is vital for maintaining healthy neural circuits. Studies led by researchers like Beth Stevens have shown that abnormal microglial activity and synaptic pruning can contribute to the onset and progression of Alzheimer’s disease.
How do microglial cells contribute to neurodegenerative diseases?
Microglial cells play a pivotal role in neurodegenerative diseases by regulating inflammation and synaptic pruning. In conditions such as Alzheimer’s and Huntington’s disease, malfunctioning microglial cells can exacerbate neuronal injury by incorrectly pruning synapses, leading to further cognitive decline. Understanding microglial function is essential for developing potential therapies for these diseases.
Who is Beth Stevens and why is her work on microglial cells significant?
Beth Stevens is a prominent neuroscientist who has significantly advanced our understanding of microglial cells and their functions in the brain. Her research has revealed how microglia are involved in synaptic pruning during development and how their dysregulation can lead to neurodegenerative diseases like Alzheimer’s. Her findings are foundational for developing biomarkers and new treatments targeting microglial dysfunction.
What is the impact of microglial cell dysfunction on the brain?
Dysfunction of microglial cells can severely impact brain health, especially in neurodegenerative diseases. When microglia mismanage the pruning of synapses, they can lead to neuronal loss and cognitive impairment, characteristic of Alzheimer’s disease. This highlights the importance of microglial function in maintaining brain homeostasis and suggests that therapeutic strategies targeting microglial activity may help mitigate neurodegenerative disease progression.
What does the term ‘brain immune system’ refer to in the context of microglial cells?
The term ‘brain immune system’ refers to the protective role played by microglial cells within the central nervous system. These cells monitor the brain for signs of injury or infection, and they respond by clearing cellular debris, which helps maintain mental and neurophysiological health. This immune function is essential for preventing neurodegenerative diseases, highlighting the therapeutic potential of targeting microglial cells in Alzheimer’s research.
How does microglia function relate to the development of Alzheimer’s?
Microglia function is directly related to the development of Alzheimer’s disease through their role in synaptic pruning and inflammation. Research has shown that improper microglial pruning can lead to an increase in neurotoxicity and an accumulation of amyloid plaques, hallmarks of Alzheimer’s. Understanding how microglia function can inform strategies to prevent or treat Alzheimer’s by modulating their behavior.
What discoveries have been made regarding microglial cells that could lead to new Alzheimer’s treatments?
Recent discoveries surrounding microglial cells, particularly those made by researchers like Beth Stevens, have unveiled their involvement in synaptic pruning and inflammation in Alzheimer’s pathology. These findings suggest that targeting microglial dysfunction or enhancing their protective capabilities may lead to innovative treatments for Alzheimer’s and other neurodegenerative diseases, providing hope for millions affected.
| Key Points | Details |
|---|---|
| Microglial Cells | Act as the brain’s immune system, cleaning up dead or damaged cells and pruning synapses. |
| Role in Alzheimer’s Disease | Aberrant pruning by microglia can contribute to neurodegenerative diseases such as Alzheimer’s. |
| Research Impact | Foundation for new biomarkers and medicines to detect and treat neurodegenerative diseases. |
| Funding and Support | National Institutes of Health and federal funding have been essential for research advancements. |
| Basic Science Importance | Basic science drives new discoveries that contribute to understanding and treating diseases. |
Summary
Microglial cells play a crucial role in brain health, acting as the immune system that protects our neural environment. The transformative research led by Beth Stevens highlights the dual nature of microglial cells; while they are essential for maintaining brain function, abnormal activity can contribute to devastating conditions like Alzheimer’s disease. Stevens’ work has paved the way for new insights into how microglia operate and their implications for neurodegenerative diseases, opening avenues for novel treatments and improving care for millions affected by Alzheimer’s.