TIM-3 Alzheimer’s treatment represents a groundbreaking advance in the fight against Alzheimer’s disease, suggesting a novel application of immune system therapy traditionally reserved for cancer treatment. Recent research has shed light on how the TIM-3 gene, specifically its role in the immune response, can help address the accumulation of amyloid plaques in the brains of individuals suffering from this debilitating condition. By targeting the TIM-3 molecule, scientists have discovered a way to unshackle brain immune cells known as microglia, allowing them to efficiently clear these harmful plaques and restore cognitive functions. This research not only highlights potential therapeutic pathways for Alzheimer’s disease but also emphasizes the interconnected nature of immune system dynamics and neurological health. With its promising results, TIM-3 therapy could pave the way for innovative strategies in treating Alzheimer’s, transforming how we manage and understand the disease.
The exploration of TIM-3 therapy in Alzheimer’s treatment brings forth an exciting avenue for addressing memory loss and cognitive decline associated with this neurodegenerative disorder. This innovative approach leverages insights from immune regulation, particularly the modulation of the TIM-3 checkpoint molecule, to enhance the ability of microglia to remove plaques obstructing neuronal function. By examining the relationships between genetic factors, such as variations in the TIM-3 gene, and the immune response, researchers are uncovering how to reactivate the brain’s natural defenses against Alzheimer’s disease. This could lead to a significant shift in how Alzheimer’s is treated, using mechanisms that have already shown efficacy in combatting cancers. As we delve deeper into understanding these links, the potential for new, effective therapies grows, inspiring hope for those affected by Alzheimer’s.
Understanding TIM-3 in Alzheimer’s Disease
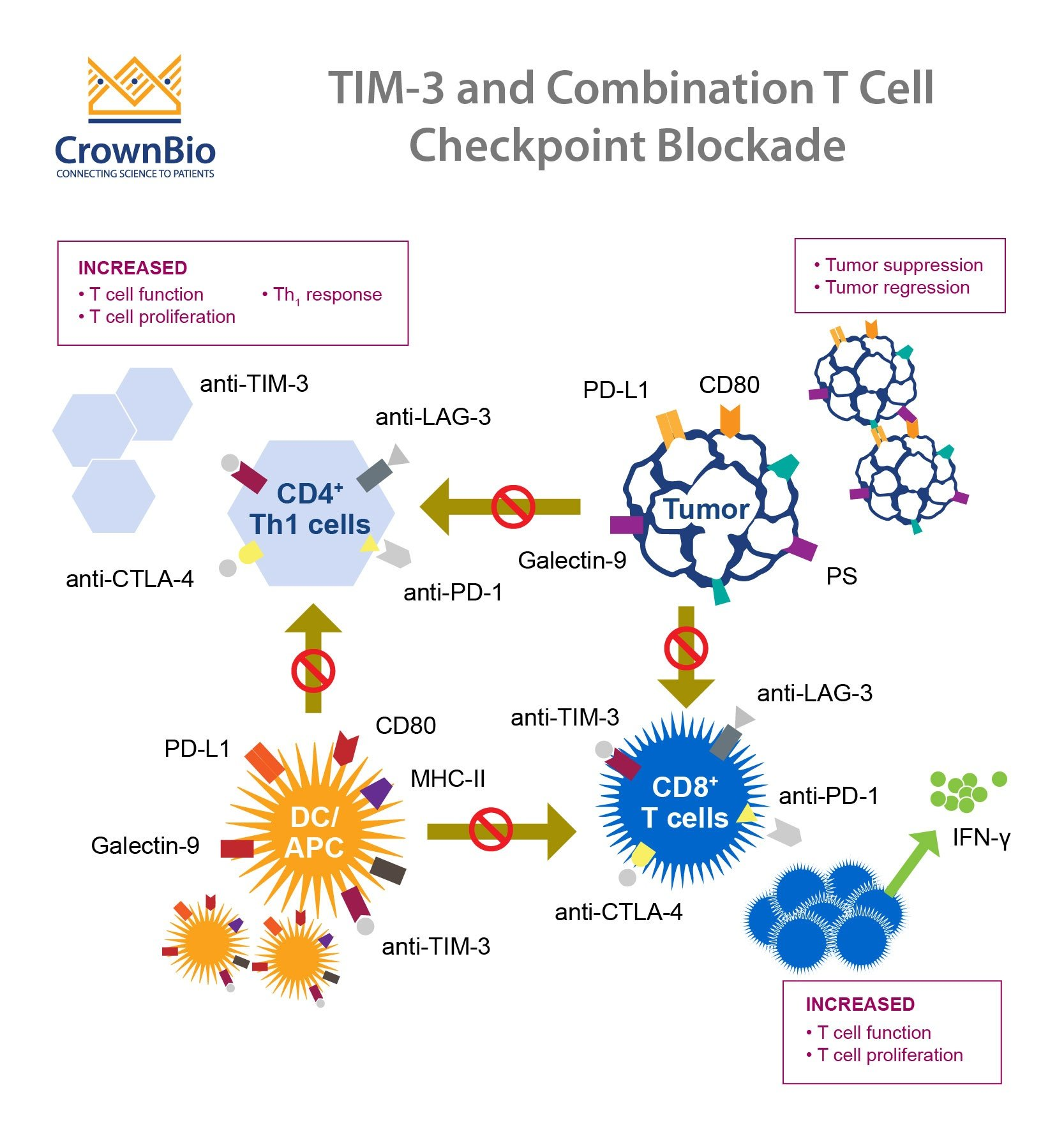
TIM-3, or T-cell immunoglobulin and mucin-domain containing-3, plays a crucial role in the body’s immune response. In the context of Alzheimer’s disease, particularly late-onset forms, TIM-3 has been identified as a significant genetic risk factor. Research has shown that a polymorphism in the TIM-3 gene correlates with the severity of Alzheimer’s manifestations. This discovery highlights the possibility of targeting TIM-3 as a therapeutic strategy to enhance the brain’s ability to combat amyloid plaques, which are notoriously detrimental to cognitive functions.
Microglia, the brain’s resident immune cells, typically function to maintain homeostasis and clear waste, including toxic protein aggregates such as amyloid-beta. However, under the influence of TIM-3, these cells can become inhibited and fail to perform their cleaning duties. The increased expression of TIM-3 on microglia in Alzheimer’s patients suggests a mechanism that prevents these immune cells from efficiently clearing plaques. By understanding this relationship, researchers are exploring ways to manipulate TIM-3 expression or function to restore the immune response against Alzheimer’s pathology.
The Role of Microglia in Alzheimer’s and Cancer Treatment
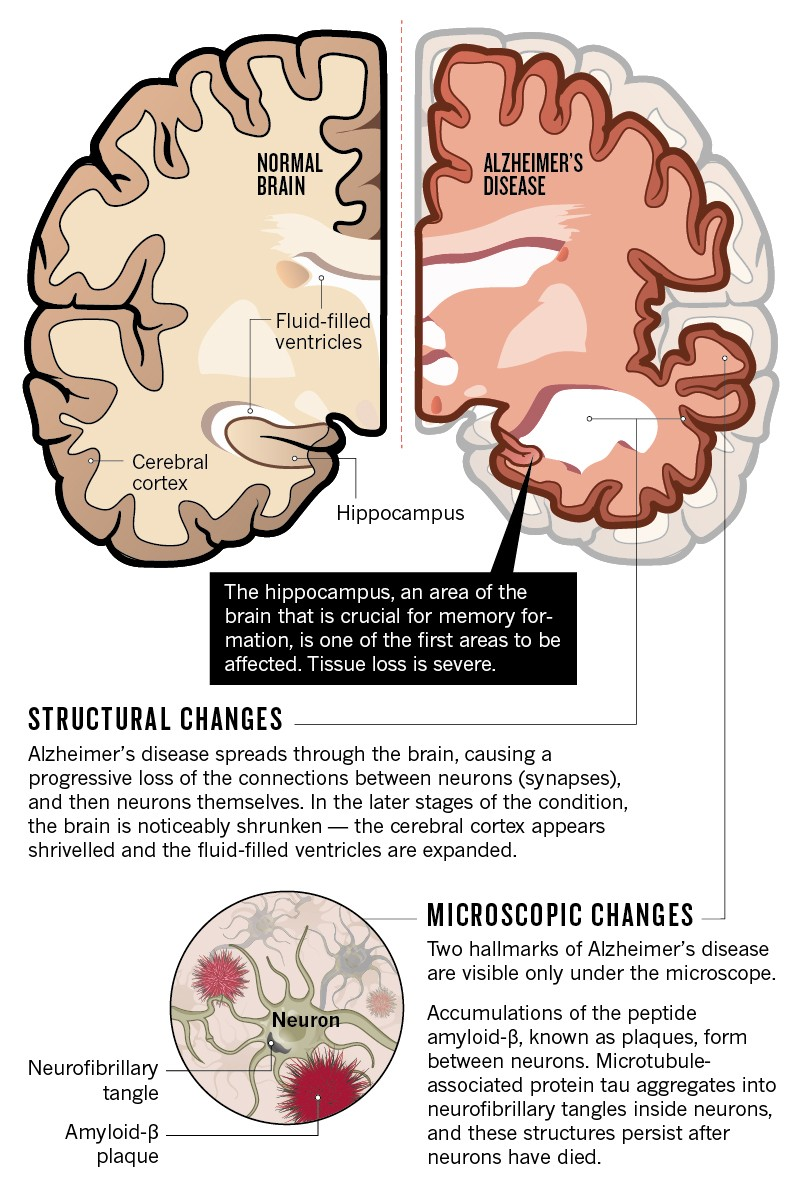
Microglia are often described as the brain’s immune system, but their role extends beyond just defending the brain from pathogens. They are responsible for synaptic pruning during the brain’s development, ensuring that neural connections are optimized for memory storage. Unfortunately, as Alzheimer’s disease progresses, microglia’s ability to prune unnecessary synapses becomes compromised, particularly when TIM-3 levels rise, leading to one of the challenges in treating the disease. Instead of cleaning up amyloid plaques, microglia may contribute to neuroinflammation and cognitive decline.
Interestingly, the immune modulation techniques used successfully against cancers, notably through checkpoint inhibitors, are now being examined for their application in Alzheimer’s treatment. Cancer therapies that inhibit checkpoint molecules like TIM-3 have been transformative, allowing T-cells to function more effectively against tumors. This insight has led researchers to investigate whether similar approaches could be applied to Alzheimer’s, potentially reactivating microglia to clear amyloid plaques and restore cognitive function.
Innovative Therapies Targeting TIM-3
Developing therapies that inhibit TIM-3 is a promising direction for Alzheimer’s treatment. Current studies are exploring the use of anti-TIM-3 antibodies or small molecules designed to block TIM-3’s inhibitory function. These therapies aim to free microglia to resume their plaque-clearing duties while preserving the brain’s essential immune balance. Given that anti-TIM-3 agents have shown success in oncology, their adaptation for Alzheimer’s represents an exciting convergence of cancer treatment strategies and neurodegenerative disease management.
Potential TIM-3 therapies highlight an innovative approach that could surpass traditional anti-amyloid treatments, which have often yielded limited results. By focusing on microglial activation and enhancing the immune response within the brain, these therapies not only target the accumulation of amyloid plaques but also aim to rejuvenate the overall cognitive landscape. The hope is that this paradigm shift in treatment could lead to meaningful improvements in Alzheimer’s patients, addressing not just symptoms, but also the underlying pathology of the disease.
Exploring Genome-Wide Associations with TIM-3
Genome-wide association studies have been instrumental in identifying genetic factors linked to late-onset Alzheimer’s disease, with TIM-3 emerging as a notable target. These studies reveal that variations in the TIM-3 gene can affect an individual’s risk of developing Alzheimer’s, underlining the interplay between genetics and brain health. Such insights enable researchers to tailor biologically relevant therapies based on genetic profiles, enhancing the likelihood of successful clinical outcomes in affected individuals.
Realizing the potential of TIM-3 as a genetic risk factor necessitates advanced research frameworks that bridge genetics with immunology. By focusing on these genetic correlations, scientists hope to uncover novel pathways and mechanisms that fuel Alzheimer’s pathology. As we develop a deeper understanding of TIM-3’s role and its interactions within the immune environment of the brain, it could lead to innovative therapies designed to modify the course of Alzheimer’s disease in genetically susceptible populations.
Potential of Anti-TIM-3 Antibodies in Alzheimer’s Treatment
The application of anti-TIM-3 antibodies in Alzheimer’s treatment marks a potential breakthrough in addressing this complex disease. These antibodies may modulate the activity of microglia, reactivating their ability to clear amyloid plaques and restore cognitive functions. While traditional Alzheimer therapies focus on targeting amyloid-beta directly, using anti-TIM-3 therapies could approach the disease from a different angle, emphasizing the role of the immune system in combating the underlying pathology.
Current trials and research involve establishing the efficacy and safety of anti-TIM-3 antibodies in preclinical models before moving towards human trials. This shift in strategy offers hope, especially after a series of setbacks in conventional Alzheimer’s drug development. By leveraging insights from immunotherapy in cancer, researchers are poised to explore transformative solutions that may turn the tide in Alzheimer’s disease treatment, utilizing targeted immune modulation to improve patient outcomes.
Cognitive Recovery through Microglial Activation
Insights from recent studies indicate that promoting microglial activation through TIM-3 inhibition can lead to cognitive improvements, at least in laboratory settings. Mice genetically engineered to lack TIM-3 exhibited remarkable recovery in memory and learning tasks after amyloid plaque clearance. This observation serves as a powerful testament to the link between immune system modulation and cognitive health, presenting a compelling narrative for developing TIM-3 targeting therapies in Alzheimer’s.
The observed improvements in cognitive behavior among TIM-3-deficient mice invite further investigation into the mechanisms of memory recovery. Researchers anticipate that unlocking the potential of microglia could also translate to humans, where enhanced immune responses may mitigate cognitive decline and restore memory function. Such predictive models shift the focus of Alzheimer’s research towards exploring how to empower the brain’s immune cells to combat neurodegenerative processes effectively.
Interaction Between TIM-3 and Neuroinflammation
The connection between TIM-3 and neuroinflammation in Alzheimer’s disease exemplifies the intricate balance within the brain’s immune response. Elevated TIM-3 levels may significantly dampen microglial activity, contributing to chronic inflammation and exacerbating neuronal damage. This inflammation is a hallmark of Alzheimer’s pathology, involving complex interactions between microglia, other immune cells, and environmental factors impacting brain health. Understanding this dynamic could open new avenues for therapeutic intervention.
Elucidating how TIM-3 influences neuroinflammatory pathways is paramount for the development of targeted Alzheimer’s therapies. By inhibiting TIM-3 in microglia, we may not only enhance their capability to clear amyloid plaques but also reduce neuroinflammation that contributes to cognitive decline. Targeting these pathways could facilitate a dual-action strategy: aiding plaque clearance while simultaneously curtailing the neuroinflammatory response, thus addressing two critical aspects of Alzheimer’s pathology.
Future Directions in Alzheimer’s Research with TIM-3
The future of Alzheimer’s research is increasingly likely to be shaped by findings related to the TIM-3 gene and its biological implications. As the characterization of TIM-3’s role evolves, researchers can anticipate a surge in novel approaches targeting this molecule. Future studies are expected to delve deeper into the genetic, immunological, and cognitive dimensions of TIM-3, paving the way for innovative treatments that holistically address Alzheimer’s disease.
By continuing to push the boundaries of our knowledge on TIM-3, researchers aim to translate laboratory findings into effective clinical strategies. Collaborative efforts, such as those seen in the current studies involving anti-TIM-3 therapies, exemplify the promising direction of combining basic science with clinical application. The goal remains clear: to develop targeted interventions that can yield significant improvements in the lives of Alzheimer’s patients, ultimately aiming for a more thorough understanding of the disease’s mechanisms and impacts.
Clinical Implications of TIM-3 Research in Alzheimer’s
The clinical implications of TIM-3 research are vast, especially regarding the development of treatments tailored to specific patient populations. With the identification of TIM-3 as a key player in the immune response of Alzheimer’s disease, clinicians can better predict which patients may benefit from immunotherapy approaches. As trials begin to assess the efficacy of TIM-3 inhibitors, these insights will inform treatment protocols and enable personalized approaches to Alzheimer’s management.
Moreover, understanding the relationship between TIM-3 and cognitive function offers a roadmap for future research endeavors. It emphasizes the importance of integrating immunology with neurodegenerative disease research, which could redefine therapeutic targets. Clinically, the successful application of TIM-3 focused therapies could not only address plaque formation and cognition but also enhance overall brain health by re-establishing optimal immune functionality.
Frequently Asked Questions
What is TIM-3 and how does it relate to Alzheimer’s treatment?
TIM-3 is an immune system checkpoint molecule that plays a significant role in the regulation of microglia, the brain’s immune cells. In Alzheimer’s treatment, studies have shown that deleting TIM-3 expression enables microglia to clear amyloid plaques, potentially improving cognitive functions in Alzheimer’s disease.
How effective is TIM-3 therapy for treating Alzheimer’s disease?
TIM-3 therapy shows promise in treating Alzheimer’s by enhancing plaque clearance in brain models. Research indicates that anti-TIM-3 antibodies can restore memory functions in Alzheimer’s mouse models, suggesting the potential for human applications in therapy.
What role do microglia play in Alzheimer’s disease treatment using TIM-3?
In the context of TIM-3 Alzheimer’s treatment, microglia are crucial as they are the primary immune cells in the brain responsible for clearing amyloid plaques. By inhibiting TIM-3, microglia can effectively attack these plaques, which is vital for restoring cognitive health in Alzheimer’s patients.
Are there any genetic links to TIM-3 and Alzheimer’s disease?
Yes, research has identified a polymorphism in the TIM-3 gene (HAVCR2) that is associated with an increased risk of late-onset Alzheimer’s disease. This genetic factor leads to higher TIM-3 expression in microglia of Alzheimer’s patients, impairing their ability to clear amyloid plaques.
What methods are being used in TIM-3 Alzheimer’s disease research?
Current methods involve genetic deletion of the TIM-3 gene in mouse models to observe effects on plaque clearance and cognitive function. This approach helps researchers understand the potential benefits of TIM-3 antagonists in treating Alzheimer’s disease.
What are the implications of TIM-3 for future Alzheimer’s treatments?
The implications of TIM-3 in Alzheimer’s treatment are significant; existing anti-TIM-3 antibodies may be repurposed to enhance microglial activity against amyloid plaques, potentially leading to more effective therapies for Alzheimer’s disease.
What challenges are faced in developing TIM-3 Alzheimer’s therapies?
Challenges include ensuring that anti-TIM-3 therapies effectively target brain microglia without causing adverse effects, as well as overcoming previous failures of Alzheimer’s treatments that aimed at amyloid beta, which often led to complications.
What can we expect from ongoing TIM-3 Alzheimer’s research?
Ongoing research aims to test humanized TIM-3 therapies in mouse models genetically engineered to reflect human disease, paving the way for future clinical trials and potential breakthroughs in Alzheimer’s treatment.
| Key Point | Details |
|---|---|
| Study Focus | Examines the potential of TIM-3 in treating Alzheimer’s using immune system strategies from cancer treatments. |
| Key Finding | Deleting TIM-3 in microglia helps clear amyloid plaques and improves memory in mice. |
| Alzheimer’s Link | TIM-3 is linked to late-onset Alzheimer’s and is expressed more in patients with a genetic polymorphism. |
| Microglia Function | Microglia are brain immune cells that clear plaques but are inhibited by TIM-3. |
| Therapeutic Approach | Potential treatments could involve anti-TIM-3 antibodies or small molecules to inhibit TIM-3 activity. |
| Research Duration | The study took five years and involved collaboration between several researchers. |
Summary
TIM-3 Alzheimer’s treatment emerges as a promising strategy that leverages immune system mechanisms traditionally used in cancer therapy. This innovative approach, which involves the deletion of TIM-3 to enhance microglial function and plaque clearance, demonstrates significant potential in early mouse models for improving cognitive function. As ongoing research continues, the repurposing of existing anti-TIM-3 antibodies may pave the way for new therapeutic options that could effectively target Alzheimer’s disease, potentially altering the treatment landscape for this challenging condition.